
This is to show that the subscript applies to the entire polyatomic ion. Write the formula for the ionic compound formed from the carbonate anion (CO32-) and the cation Ca2+. Quiz Course 39K views Calcium Ion Formula A calcium ion has a net ionic charge of + 2. Give the chemical formula for calcium bisulfate. the number of ions or ppm as calcium carbonate: CaCO3). Write the formula for the compound calcium cyanide.
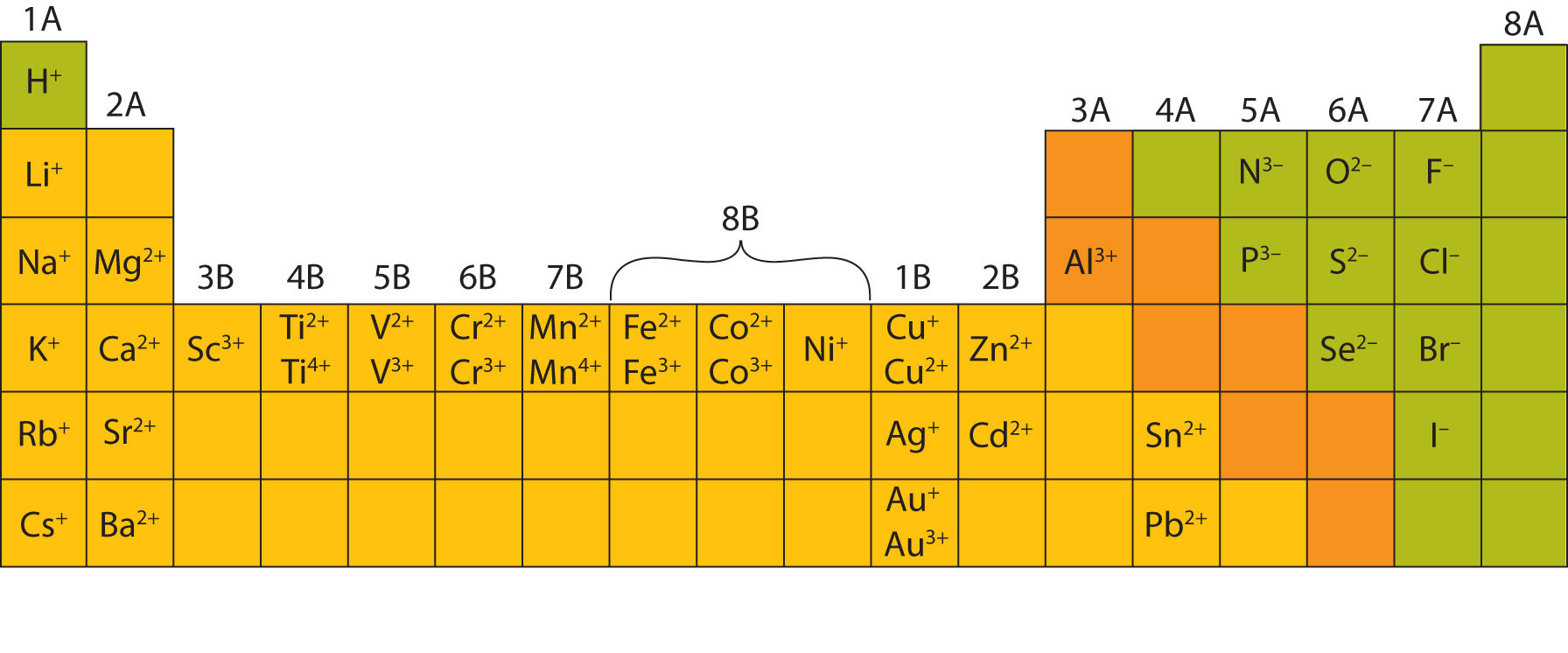
If more than one of a particular polyatomic ion is needed to balance the charge, the entire formula for the polyatomic ion must be enclosed in parentheses, and the numerical subscript is placed outside the parentheses. What is the chemical formula of the carbonate ion Write the formula and charge of the carbonate ion. The rule for constructing formulas for ionic compounds containing polyatomic ions is the same as for formulas containing monatomic (single-atom) ions: the positive and negative charges must balance. calcium carbonate ) than calcite ( rhombohedral structure ). calcium and bromine combine, they form the ionic compound. The effects of polyelectrolyte charge density and suspension ionic strength were investigated using PCC as model of polydispersed colloid. charge due to electrostatic interactions between Ca and dodecyl sulfate. Calcium Bromate Ionic Or CovalentCalcium carbonate (CaCO 3) has ionic bonding between calcium. 2011 Dec 1 84(11):1234-1242.\): Some Polyatomic Ions Name We quantified the adsorption kinetics of cationic polyacrylamides (CPAMs) from suspension onto precipitated calcium carbonate particles (PCCs) and its effect on the PCC flocculation dynamics. Treatment and prevention of kidney stones: An update. Calcium consumer fact sheet.įrassetto L, Kohlstadt I. National Institutes of Health Office of Dietary Supplements. Vitamin D, calcium, or combined supplementation for the primary prevention of fractures in community-dwelling adults: Preventive medication. Consequences of inadequate intakes of vitamin A, vitamin B12, vitamin D, calcium, iron, and folate in older persons.
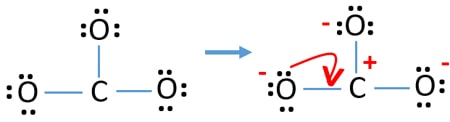
Relative bioavailability and pharmacokinetic comparison of calcium glucoheptonate with calcium carbonate. Wiria M, Tran HM, Nguyen PHB, Valencia O, Dutta S, Pouteau E. Calcium fact sheet for health professionals. It can also be used as cosmetics fillers. Since both have the charge with magnitude of 2, it is written as C a C O 3 when simplified.

That +2 charge is a result of calcium having 2 valence electrons that get donated to another ion to make a.
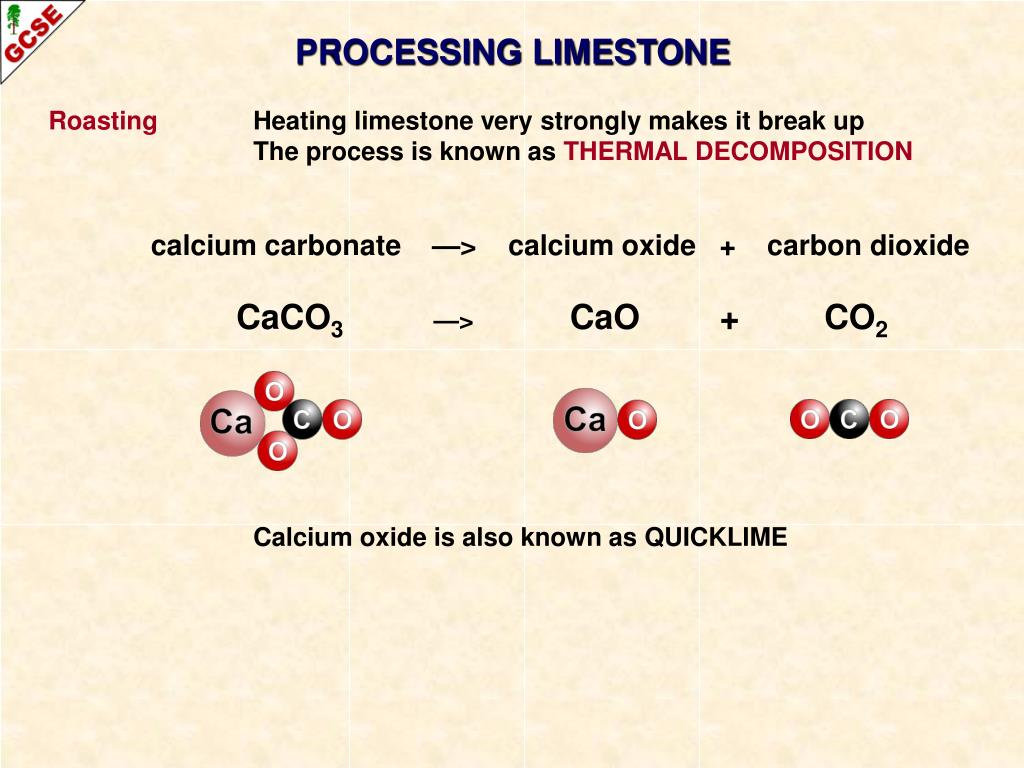
Using the criss-cross rule, the charge of the ion will be the subscript of the counterion. Calcium can most commonly be found in its ionic form, however, which carries a +2 charge. But CaCO3 contains covalent bond also as (CO3)2- is made from carbon and oxygen atoms held together by covalent bond. The calcium ion and carbonate ions are held together by an ionic bond. The cation is calcium ion Ca2+ and anion is carbonate ion (CO3)2. Medicinally, we use it as an antacid or a calcium supplement. It is composed of a calcium ( C a 2 + ) cation and a carbonate ( C O 3 2 ) anion. CaCO3, is an ionic compound made up of cation and anion. It is a white and insoluble powder-like substance that occurs naturally in minerals, marble, chalk, limestone, shells, calcite, pearl, and other related compounds. Formula and structure: The calcium carbonate chemical formula is CaCO 3 and its molar mass is 100.0869 g mol-1.The molecule is formed by the calcium cation Ca +2 and the carbonate anion CO 3-2. Our findings support the fact calcium carbonate surface is neutral and ions compete for adsorption in -plane. The total electrical charge of the nucleus is therefore +Ze, where e (elementary charge) equals to 1,602 x 10-19 coulombs. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z. Dietary Reference Intakes for Calcium and Vitamin D. Introduction The term 'carbonate' is usually used to refer to one of its salts or carbonate minerals. Calcium Carbonate is a chemical compound having the chemical formula CaCO3. Calcium is a chemical element with atomic number 20 which means there are 20 protons in its nucleus. Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium.


 0 kommentar(er)
0 kommentar(er)
